Read on to find out the name of the element with the longest name. We’ll also cover some frequently asked questions about the element.
Element With The Longest Name

Rutherfordium(Rf) is the element with the longest name. It contains 13 letters.

| Mass number | [267] |
| Atomic number | 104 |
| Group | group 4 |
| Period | period 7 |
| Block | d-block |
| Electron configuration | configuration [Rn] 5f14 6d2 7s2 |
| Electrons per shell | 2, 8, 18, 32, 32, 10, 2 |
| Phase at standard temperature and pressure | solid (predicted) |
| Melting point | 2400 K (2100 °C, 3800 °F) |
| Boiling point | 5800 K (5500 °C, 9900 °F) |
| Density | 17 g/cm3 |
| Oxidation states | (+2), (+3), +4 |
| Ionization energies | 1st: 580 kJ/mol 2nd: 1390 kJ/mol 3rd: 2300 kJ/mol |
| Atomic radius | empirical: 150 pm |
| Covalent radius | 157 pm |
| Natural occurrence | synthetic |
| Crystal structure | hexagonal close-packed 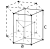 |
| CAS Number | 53850-36-5 |
Source: Wikipedia
Georgy Flerov and colleagues found Rutherfordium in 1964 in Dubna, near Moscow, Russia, while Albert Ghiorso and colleagues discovered it independently in 1969 in Berkeley, California, USA.
It was named after the British physicist Ernest Rutherford, who was born in New Zealand.

Rutherfordium was reportedly discovered for the first time in 1964 at the Joint Institute for Nuclear Research in Dubna (a town in Moscow Oblast, Russia.)
There was much debate over the name, with Russian scientists proposing kurchatovium and American scientists proposing rutherfordium for the new element. The teams participating reached an agreement in 1997 and adopted the present name Rutherfordium.
Rutherfordium is a synthetic element, meaning it does not exist naturally on Earth and is produced in a laboratory.
It is radioactive, with the most stable known isotope, 267Rf, having a half-life of approximately 1.3 hours or around 80 minutes.
Rutherfordium can be created by the nuclear fusion of plutonium-242 with neon ions in a nuclear reactor. It can also be produced by bombarding californium-249 with carbon ions.
Rutherfordium Location On The Periodic Table
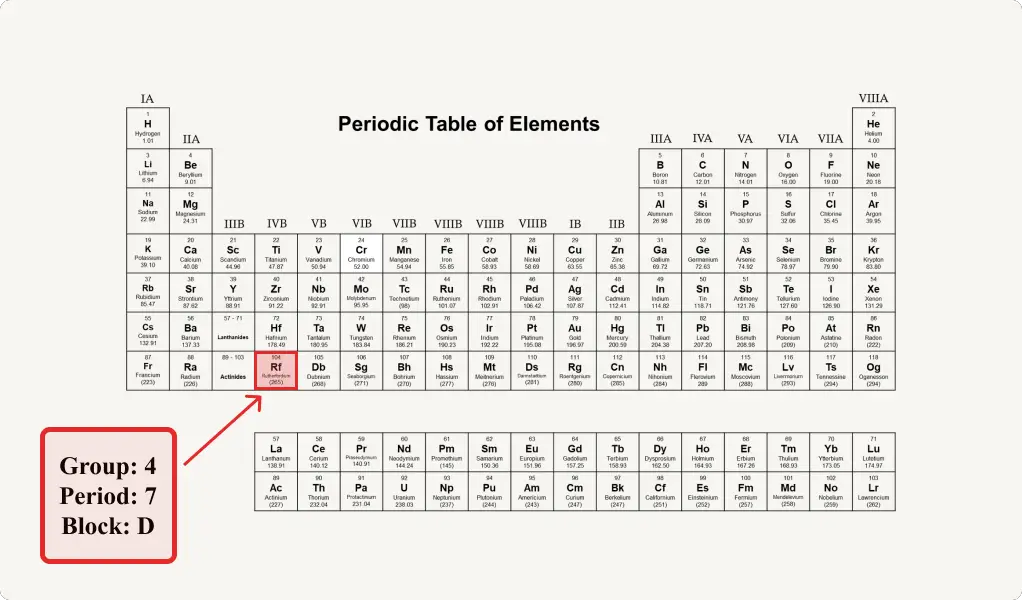
Group: 4, Period: 7, Block: d
Rutherfordium is a d-block element that belongs to the 7th period and group 4 of the periodic table.
Name An Element With The Longest Name
Rutherfordium is the element with the longest name.
What Is The Longest Element Name On The Periodic Table?
Rutherfordium is the longest element name on the periodic table.
Element With The Most Letters
The element with the most letters is Rutherfordium. It contains 13 letters.
Chemical Element With The Longest Name
Rutherfordium is the chemical element with the longest name.
What Is Rutherfordium Used For?
Rutherfordium is currently only used in research and has no recognized biological purpose.
Who Discovered Rutherfordium?
Georgy Flerov and colleagues found Rutherfordium in 1964 in Dubna, near Moscow, Russia, while Albert Ghiorso and colleagues discovered it independently in 1969 in Berkeley, California, USA.
How Many Protons Does Rutherfordium Have?
Rutherfordium has 104 protons.
Is Rutherfordium Radioactive?
Yes, Rutherfordium is radioactive.
How Many Electrons Does Rutherfordium Have?
Rutherfordium has 104 electrons.
When Was Rutherfordium Discovered?
Rutherfordium was discovered in 1964 at the Joint Institute for Nuclear Research in Dubna (a town in Moscow Oblast, Russia.)
How Much Does Rutherfordium Cost?
Rutherfordium is not for sale and is exclusively used for research purposes.
What Is Rutherfordium Atomic Mass?
Rutherfordium has an atomic mass of 267 g.mol -1.
What Is Rutherfordium’s Atomic Number?
Rutherfordium’s atomic number is 104.
When Was Rutherfordium Named?
Rutherfordium was officially named in 1997.
How Many Rutherfordium Isotopes?
Rutherfordium has no stable isotopes.
How Many Neutrons Does Rutherfordium Have?
Rutherfordium has 157 neutrons.